Gay-lussac's Law Assumes That __________.
Learning Objective
- State Charles' Police and its underlying assumptions
Key Points
- The lower the pressure level of a gas, the greater its volume (Boyle's Law); at low pressures,
volition accept a larger value. - Charles' and Gay-Lussac'south Police can be expressed algebraically as
or
.
Terms
- accented zerothe theoretical lowest possible temperature; by international agreement, absolute zero is divers equally 0 K on the Kelvin scale and as −273.15° on the Celsius calibration
- Charles' lawat constant pressure, the volume of a given mass of an ideal gas increases or decreases past the aforementioned cistron as its temperature on the absolute temperature scale (i.due east. gas expands every bit temperature increases)
Charles' and Guy-Lussac's Police
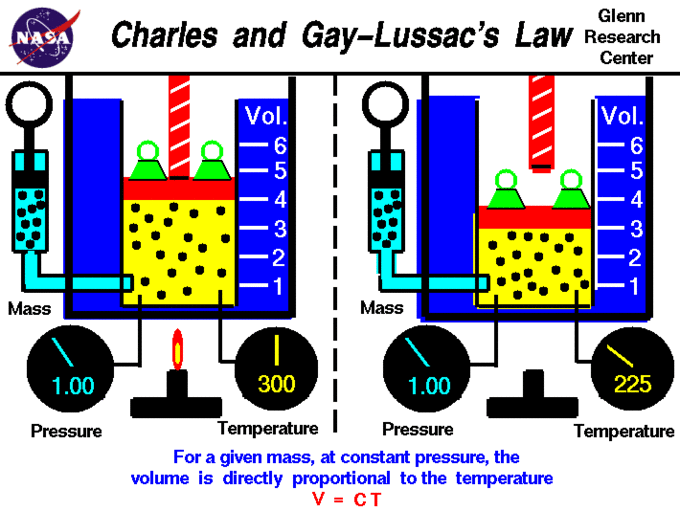
Charles' Constabulary describes the relationship betwixt the volume and temperature of a gas. The police was first published by Joseph Louis Gay-Lussac in 1802, simply he referenced unpublished work by Jacques Charles from effectually 1787. This law states that at constant pressure, the book of a given mass of an ideal gas increases or decreases past the same factor every bit its temperature (in Kelvin); in other words, temperature and volume are direct proportional. Stated mathematically, this human relationship is:
Example
- A car tire filled with air has a volume of 100 L at 10°C. What will the expanded volume of the tire exist after driving the auto has raised the temperature of the tire to forty°C?
-
-
-
V vs. T Plot and Charles' Law
A visual expression of Charles' and Gay-Lussac'due south Law is shown in a graph of the volume of i mole of an ideal gas as a function of its temperature at various constant pressures. The plots show that the ratio
(and thus
) is a constant at any given pressure. Therefore, the law can be expressed algebraically equally
or
.
Extrapolation to Goose egg Volume
If a gas contracts by ane/273 of its volume for each caste of cooling, information technology should contract to zero volume at a temperature of –273°C; this is the lowest possible temperature in the universe, known as absolute zilch. This extrapolation of Charles' Law was the get-go evidence of the significance of this temperature.
Why Do the Plots for Different Pressures Accept Unlike Slopes?
The lower a gas' pressure, the greater its volume (Boyle's Law), and so at low pressures, the fraction \frac{5}{273} will have a larger value; therefore, the gas must "contract faster" to reach goose egg volume when its starting book is larger.
Show Sources
Licenses and Attributions
Gay-lussac's Law Assumes That __________.,
Source: https://www.coursehero.com/study-guides/introchem/charles-and-gay-lussacs-law-temperature-and-volume/
Posted by: lopezbeturped1953.blogspot.com


0 Response to "Gay-lussac's Law Assumes That __________."
Post a Comment